ThromBot™
ThromBot™ is a breakthrough radial-access compatible thrombectomy device that rapidly clears medium vessel occlusions (MeVOs)..
-
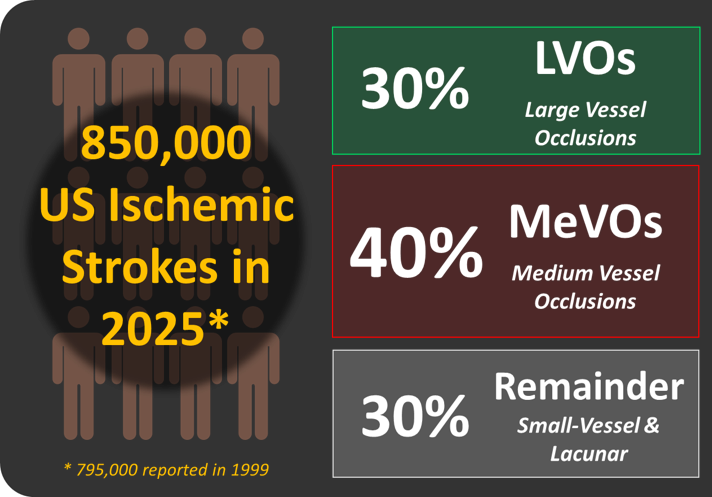
The Problem
More than 300,000 U.S. patients each year present with distal MeVOs in small, tortuous cerebral arteries that current thrombectomy devices cannot safely reach. These patients face delayed or incomplete reperfusion, higher disability and mortality, and greater lifetime care costs. Because approved tools are large and stiff, they increase small-vessel injury risk and must rely on femoral access, the latter of which is associated with more complications and higher hospital costs
-
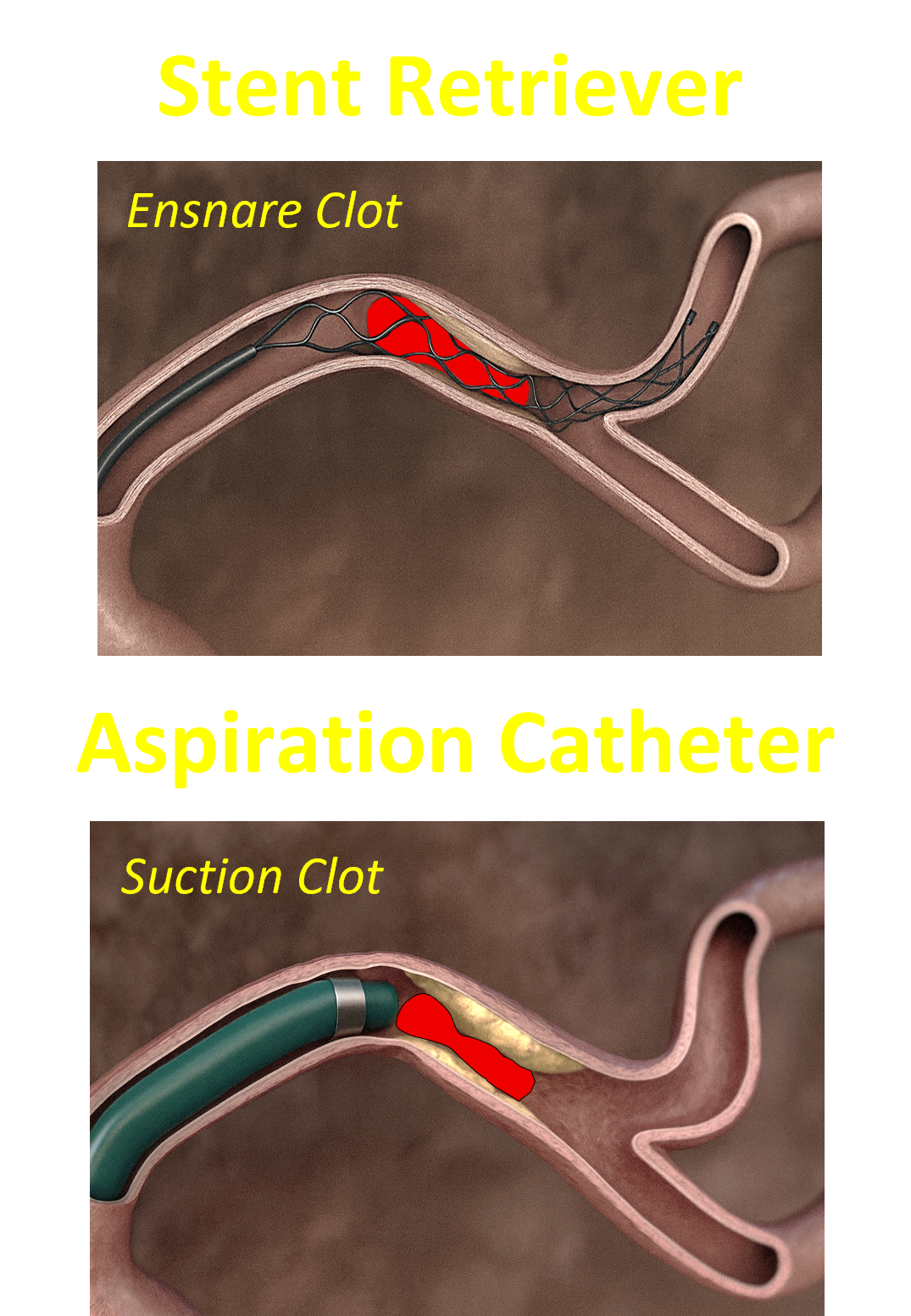
The Need
Because approved thrombectomy tools are large and stiff, they increase small-vessel injury risk and must rely on femoral access, the latter of which is associated with more complications and higher hospital costs. Taken together, a safer, more trackable, small profile thrombectomy solution is needed to reliably restore distal perfusion while reducing complications and cost.
-
The Solution
ThromBot™ is a thrombectomy system being developed by UNandUP. Only limited information is publicly available.
Once proven, ThromBot™ is intended to be the first thrombectomy device that can safely and effectively clear clots associated with MeVOs. Key benefits include:
For MeVO patients – Improved cerebral reperfusion and better neurological outcomes. Support for safer, lower-complication radial access.
For physicians – A smaller, more trackable device designed to access MeVOs and aspirate embolic debris. Integrated 3D localization, flow sensing, and stall/force sensing to support safer and more effective procedures.
For hospitals and payers – An increase in the number of treatable stroke cases. Improved stroke-related revenue for hospitals and shorter lengths of stay, helping lower overall cost of care.
For health systems – Extension of the proven benefits of thrombectomy to a larger population of stroke patients.